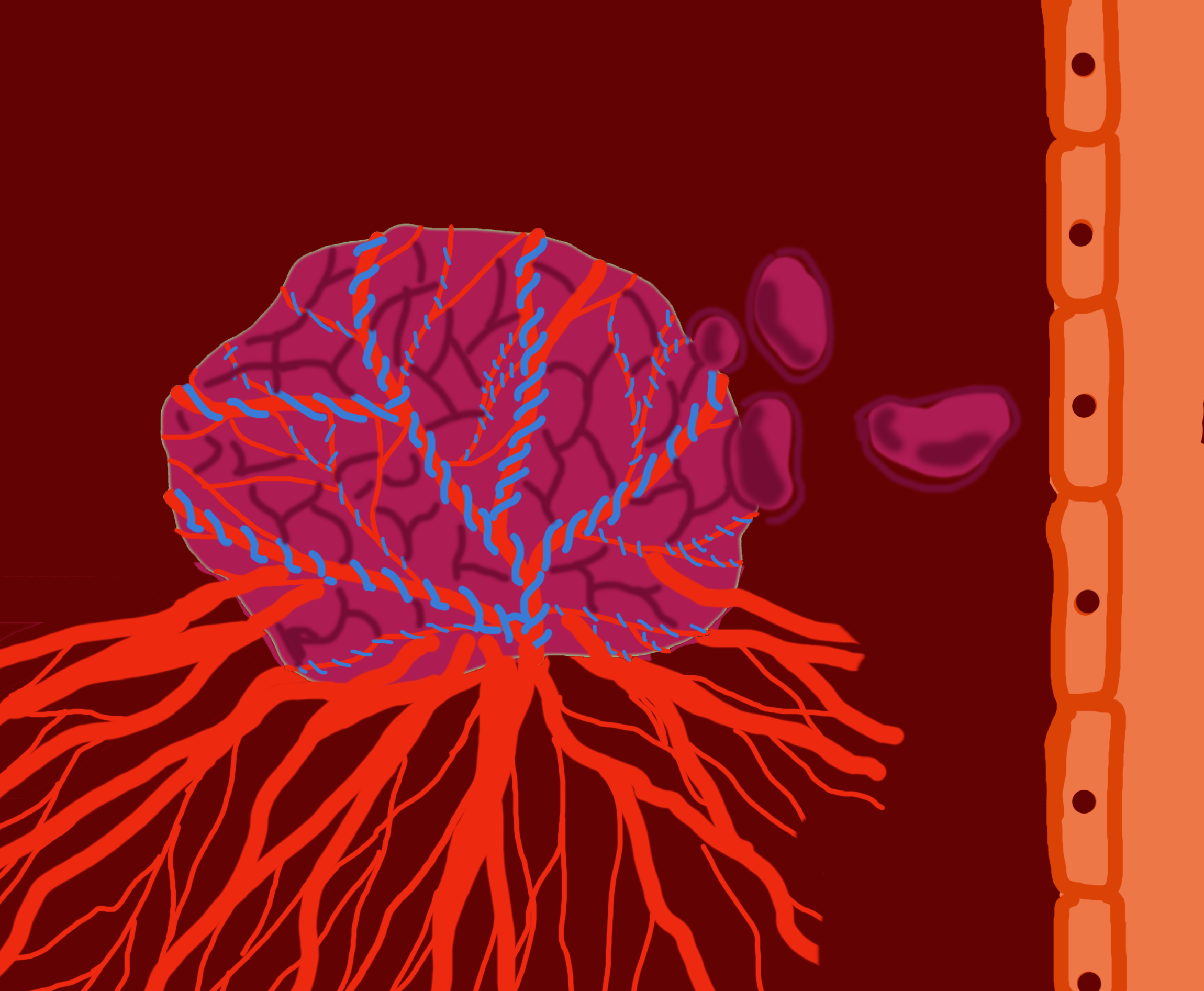
Researcher discovers cancer may not be forming in the one way we previously thought
A development in cancer research has been made by researchers from Montreal and London, England. They identified some cancer cells have the ability to co-opt cells—meaning they can obtain nutrients and oxygenation from pre-existing blood vessels—rather than needing to create their own blood vessels for fuel and growth.
Thirty-two researchers from both the Research Institute of the McGill University Health Centre
(RI-MUHC) and the Institute of Cancer Research in London made the discovery, and published their findings in a report titled “Vessel co–option mediates resistance to anti–angiogenic therapy in liver metastases” in the scientific journal Nature Medicine.
“It’s well accepted that when cancer grows, it requires more nutrients and oxygen—which means they require more blood vessels,” said Dr. Peter Metrakos liver surgeon, a member of RI-MUHC, lead author of the study and director of both the Multi-Organ Transplant Program and of Hepatopancreatobiliary Surgery. He said in order for cancer to grow, new blood vessels have to grow into the tumour. Therefore, he said in order for tumours to grow, cancer cells had to produce their own blood cells. He said the new vessel growth in the tumour is called angiogenesis, treatable with antiangiogenic therapies.
Metrakos said for the last 30 to 40 years, antiangiogenic drugs have been under development to target some of these factors. “But the problem has been that the [treatment] results are mixed—it’s not as good as we thought it was going to be.”
He said he believes that if he and his team can stop the new blood vessels from growing into the tumour, the tumour will not continue to gain proper nutrition and lose the ability to sustain itself. Metrakos said while some tumours do produce new blood vessels, it turns out not all of them do. He said he and the team discovered some kinds of tumours obtain nutrients and oxygen by co-opting existing blood vessels in the body.
“So rather than producing new blood vessels, [the cancer cells] kind of sneak their way around a tumour or around a blood vessel and co-opt it and then get their nutrients and oxygenation from blood vessels that’s existing,” said Metrakos. He said this explains why therapies that target new blood vessel development have had mixed results—co-opting cancer cells don’t produce new blood vessels, so there is nothing for the treatment to target.

In Metrakos’ research on how cancer evolves in the liver, he said he has found that approximately 40 to 45 per cent of tumours acquire their blood supplies through new blood vessels, while an additional 40 to 45 per cent obtain their blood supply by converting existing blood vessels from the liver. He said this clarifies why current treatments that aim for new blood vessels are not as successful as it was thought.
According to Metrakos, this is reflected in the survival rate following tumour-removal surgery, also known as resection. When patients were treated with antiangiogenic therapy and then had a resection, they had a less than 20 per cent survival rate if their cancer had been co-opting, compared to the 50 per cent survival rate of patients with a cancer that produced its own blood vessels.
Metrakos said he can determine whether the tumour is creating new cells or co-opting cells by staining an organ’s blood vessels. He said he has preliminary data that will be able to identify these different types of tumours using imaging with an MRI and a CT scan—but the research is in its beginning stages.
What the researchers have found through the use of an animal model, is by decreasing the motility of an animal via genetically manipulating cancer cells, they find the cancer cannot co-opt blood vessels. This forces the cancer to develop new blood vessels, which can then be targeted with the traditional method of antiangiogenics.

“That’s an experimental model, and it could pan out in human beings, but it could take a little time,” said Metrakos. For now, this theory has only been tested on animals.
“While we assume that any solid tumour over a certain size needs anti-angiogenesis to stimulate these vessels, their group has found there are a lot of tumours that aren’t doing this,” said Alisa Piekny, an associate professor for Concordia’s biology department.
“What would be really important to do is that each patient would have to be treated as a different case and you would have to get that information about their tumour before you start treating them with angiogenic drugs so that you could tailor the treatment more specifically to them,” said Piekny.
Piekny’s cancer research involves understanding the basic mechanism underlying cell division, and testing compounds that may target cell division and could be used in cancer treatment.
Piekny said this new development in cancer treatment reflects an overall change in the way researchers understand cancer. “You can’t treat [all cancer] the exact same way,” she said. She said insights like Metrakos’ helps improve our understanding of these different types of cancers. However, find the cure for cancer will not be an overnight discovery.
“Now we have a way to attack maybe a subset of cancers that do what they found where they can use the healthy vessel instead,” she said. “We’re going to treat those ones a little bit differently and we can improve the success rate, maybe.”
Piekny has a team of Concordia students that conduct research on cancer at the Loyola campus—which includes three PhD students, a masters student, multiple undergraduate students and visiting scientists. The undergrads switch out every couple of terms, the masters student work for usually two years at a time, while PhD’s work for approximately four years.
When asked how long he had been working on this research, Metrakos chuckled. “I’ve been preparing my whole life for this,” he said. He said he’s been working on this for four and a half years. “Obviously it’s going to be another ten years of work to get this to where we want it to go.”